Abstract
Background: Recipients of HLA mismatched unrelated donor (MMURD) hematopoietic cell transplant (HCT) are at increased risk for severe acute graft versus host disease (aGVHD) and treatment related mortality (TRM). Abatacept, CTLA4-Ig was granted FDA approval for the prevention of aGVHD in patients receiving unrelated donor HCT in part based on results of ABA2, a phase II trial evaluating abatacept in addition to standard immunoprophylaxis (calcineurin inhibitor (CNI)/ methotrexate (MTX) for GVHD prevention in either 8/8 MUD or 7/8 MMURD HCT (Watkins et al., 2021). The 7/8 MMURD cohort from ABA2 showed a significant reduction in grade III-IV aGVHD compared with a matched cohort from the CIBMTR receiving CNI/MTX alone (2.3% vs 30.2%, P<0.001). The lower rates of severe aGVHD translated into significant improvements in TRM and overall survival (OS) at 2 years for patients receiving abatacept. While ABA2 included pediatric and adult patients in the analysis, outcomes were not reported separately. Furthermore, there may be selection bias associated with study eligibility and participation that could result in benefits that may not translate into everyday clinical practice.
Objectives: To determine the real-world impact of abatacept combined with CNI/MTX on the development of acute and chronic GVHD, survival, and relapse in pediatric and adult patients with hematologic malignancies receiving of 7/8 MMURD HCT as standard of care (off-study).
Methods: We conducted a multi-center retrospective analysis of outcomes for pediatric and adult patients receiving a 7/8 MMUD HCT (bone marrow (BM) or peripheral blood stem cells (PBSC)) for hematologic malignancies and received GVHD prophylaxis with abatacept (4 doses on days -1, +5, +14, and +28) combined with CNI/MTX between January 2015 and December 2021. Patients were excluded if they were on ABA2, not in remission at time of HCT, received a prior HCT, or required additional GVHD prophylaxis. The analysis included consecutive adult patients transplanted at Emory University and pediatric patients transplanted at Children's Healthcare of Atlanta and Boston Children's Hospital. Data were analyzed for all patients and for pediatric and adult patients as separate cohorts.
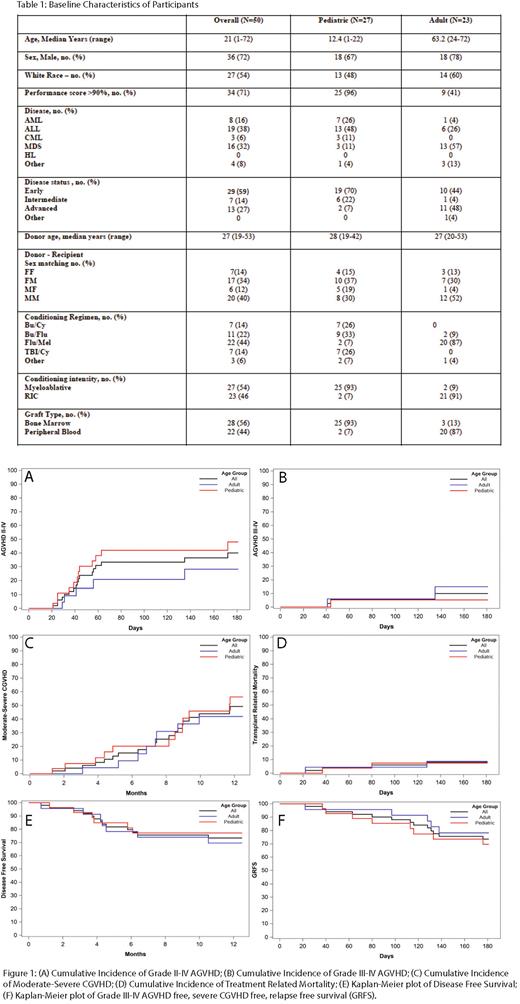
Results: A total of 50 consecutive patients (27 pediatric patients and 23 adult patients) met inclusion criteria. Median follow up was 759 days. Twenty-five of 27 pediatric patients received BM grafts while 20 out of 23 adult patients received PBSC. Grade II-IV aGVHD at day 180 was 40%, 48% and 28% for all patients, pediatric patients and adult patients, respectively. Grade III-IV aGVHD at day 180 was 10%, 5% and 15% for all, pediatric and adult patients respectively. Moderate-severe chronic GVHD (cGVHD) at 1 year was 49%, 56% and 42% for all, pediatric and adult patients respectively. TRM at day 180 was 8%, 7% and 9% for all, pediatric and adult patients respectively. OS at 1 year was 82%, 85% and 78% for all, pediatric and adult patients respectively. Relapse at 1 year was 16%, 15% and 17% for all, pediatric and adult patients respectively Disease-free survival (DFS) at 1 year was 73%, 77% and 70% for all, pediatric and adult patients respectively. Grade III-IV aGVHD free, severe cGVHD free, relapse free survival at day 180 was 74%, 70% and 78% for all, pediatric and adult patients respectively.
Conclusion: Our real-world results using abatacept prophylaxis for 7/8 MMUD HCT compared favorably with ABA2 results with low incidence of grade III-IV aGVHD and TRM and encouraging DFS. These results are promising given that our off-study patients had more advanced disease compared to the patients on ABA2 (27% vs 13%). This was especially true in our adult patients where 50% had advanced disease and almost all received PBSC grafts. This study was the first to analyze and report outcomes separately for pediatric and adult patients receiving abatacept for GVHD prophylaxis. Our results indicate that both pediatric and adult patients had favorable outcomes with abatacept. Similar to results from ABA2, cGVHD rates were not reduced with the 4 dose abatacept regimen and further studies are needed to evaluate the efficacy of extended dosing abatacept to mitigate this risk. Our real-world results mirrored the results from ABA2 and suggest that the addition of abatacept to standard immunoprophylaxis for our highest risk patients can be effective at substantially improving aGVHD related transplant outcomes in MMUD recipients.
Disclosures
Kean:Bristol Myers Squibb: Research Funding; Vertex: Consultancy; Magenta: Research Funding; HiFiBio: Consultancy; Mammoth Biosciences: Current equity holder in private company; EMD-Serono: Research Funding; Kite/Gilead: Research Funding; Bluebird Bio: Research Funding. Qayed:Novartis: Honoraria; Vertex: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal